
Digital Health Regulatory: FDA Precert
The US Food and Drug Administration just published an early draft for comment on Developing Software Precertification Program: A working Model. After launching the Precert program in January 2018, their timeline is to produce versions of the working model throughout the year and publish the final model in December. The current draft of the working model covers:
- the program goal, vision and scope
- excellence appraisal and precertification
- review pathway determination
- streamlined premarket review process and
- real-world performance
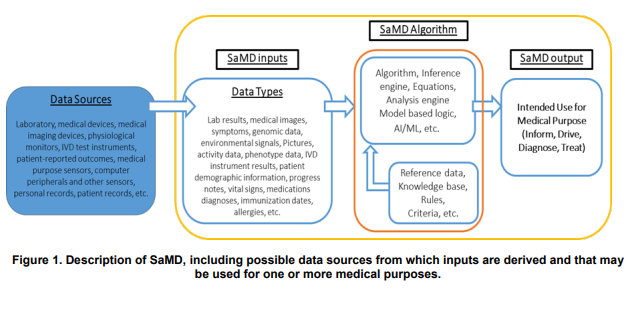
The document addresses the many challenges of regulating Software as a Medical Device (SaMD), software for a medical purpose without being part of a hardware medical device. It acknowledges the fact that software is often modified after it is first released and may have multiple data sources and data types which are processed through an algorithm, with associated reference data to produce an output for medical purposes.
The program concept is looking to software manufacturers who have demonstrated a culture of quality and organizational excellence who meet five excellence principles:
- Product quality
- Patient safety
- Clinical responsibility
- Cybersecurity responsibility
- Proactive culture
There is also a strong emphasis on Real World Performance Analytics for monitoring safety and effectiveness of the SaMD product.

This working model follows the description published in BMJ editorial in January 2018: Digital healthcare: regulating the revolution. “The tension between commercialisation and regulating for patient safety is clear from the FDA proposals, but it’s easy to see why the US regulator wants to minimise the types of technology requiring premarket review. Products are being developed so fast that enormous resources would be required to keep pace.” In a webinar about the working model on June 27, Bakul Patel, FDA Senior Policy Advisor, strongly encouraged those in the industry to submit comments on the public docket.




