Focusing on a Single Disease and New Technology Leads to FDA Approval
Heart disease still is the leading cause of death in the US and heart failure hospitalizations cost the US $26 billion per year. Re-hospitalization is the cause of much of this expense – 25% of patients are readmitted within 30 days.
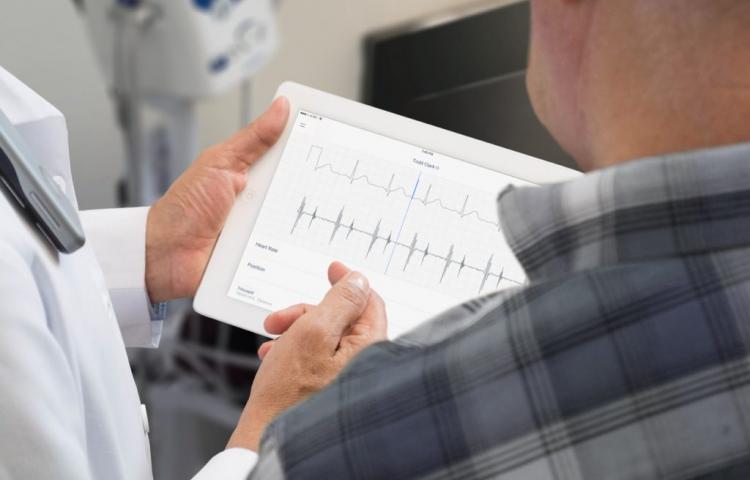
To address this growing challenge, Eko DUO combined new cardiology-grade sensors and advanced medical engineering technology to create a connected health device that monitors heart sounds and ECG, enabling quick diagnosis and remote monitoring. Their success through use at institutions like Mass General and UCSF has led to FDA approval.
The challenge of going from concept to FDA approve requires this kind of focus – solving a specific medical issue which produces both good medical outcomes and addresses financial incentives. One set of tools which can help in the process are the Continua Design Guidelines and the Continua Playbook. Both are available from the Personal Connected Health Alliance.




