
How to Leverage a Continua Certified Reference System
Published by the Personal Connected Health Alliance (PCHAlliance), a Continua Reference System is a certified product that may be used by any other organization to create their own Continua certified product. The purpose of a Reference System is to enable Company A, typically an Original Equipment Manufacturer (OEM), to showcase a product that Company B may later re-brand or improve to achieve their own product listing on the Continua Certified Product Showcase (CPS). Today, the Showcase has over 50 Reference Systems certified.
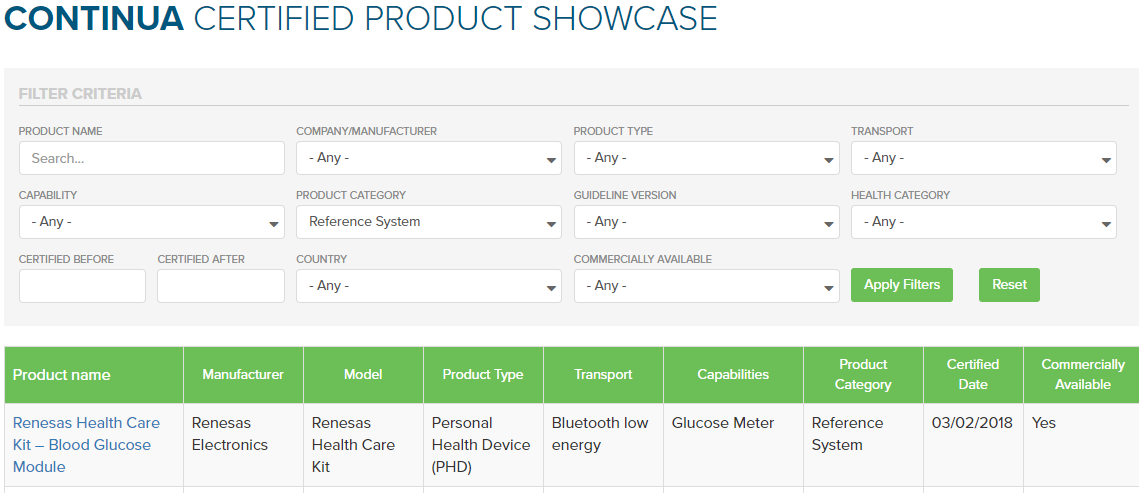
One very recent example of a Reference System is the Renesas Healthcare Meters Kit Modules were certified in early 2018, It consists of a blood pressure, heart rate/pulse-ox, and blood glucose modules. The Renesas Healthcare Meters Kit is based on the Renesas Synergy™ S3 Series of micro-controllers units (MCUs are a computer on a single chip), a low-power Intelligent Bluetooth Smart RL78/G1D MCU, and a USB Charger IC. As the modules in this kit are Continua certified, any other organization may reuse these Renesas modules to create their own Continua certified product. It can be as simple as re-branding the product with their own name.
To find Certified Reference Systems via the Certified Product Showcase, just select the ‘Product Category’ column to filter the list of Certified products, then scroll down to ‘Reference Systems’. (Alternatively, you can export a CSV spreadsheet of the entire listing at the bottom left of the page).
If your organization is interested in certifying one of its personal connected health solutions as a Continua Reference System, please see our Conformity Assessment Test Plan (CATP) document which explains the requirements in detail (see page 10).




